Chinese Medicine Regulatory Office
Site Menu
Notices
What's New
For latest Government press release, you may visit the HKSAR website.
- The 1st to 5th monographs of project “Macroscopic and Microscopic Identification of Cortex type of Decoction Pieces Commonly Found in Hong Kong” have been published in January 2025 (27 January 2025)
- DH publishes Hong Kong Chinese Materia Medica Standards Volume 11 (30 December 2024)
- New analytical methods of project “Analysis of Chemical Markers in Proprietary Chinese Medicines Containing Psoraleae and Ginseng” have been published in December 2024 (30 December 2024)
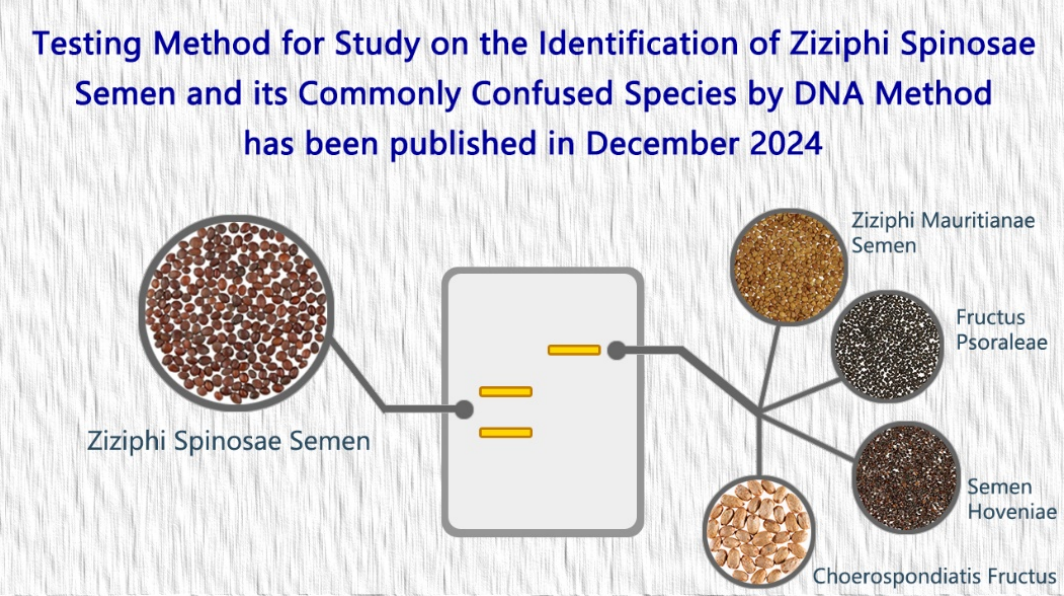
- A new analytical method of the project “Study on the Identification of Ziziphi Spinosae Semen and its Commonly Confused Species by DNA Method” has been published in Dec 2024 (20 December 2024)
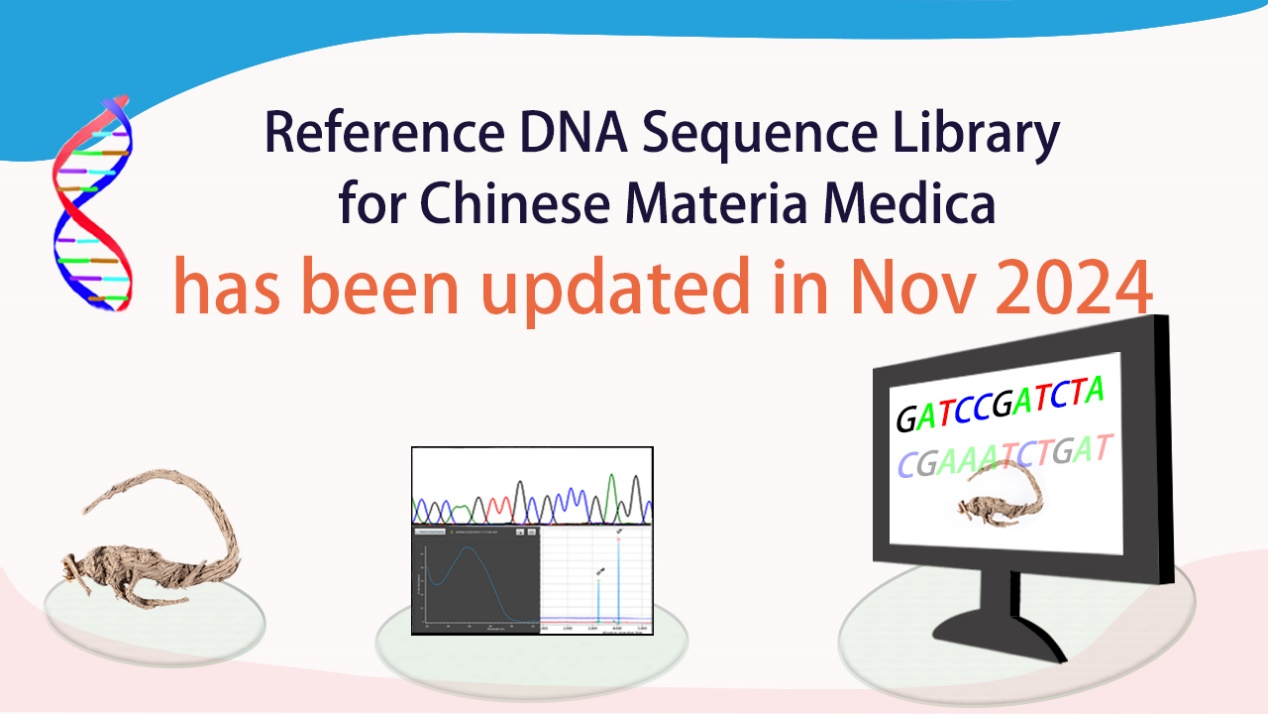
- The reference DNA sequence library of project “Reference DNA Sequence Library for Chinese Materia Medica (CMMRSL)” has been updated in November 2024 (8 November 2024)
- DH signs Co-operation Arrangement on Research for Quality Testing and Standards of Chinese Medicines with Experimental Research Center of the China Academy of Chinese Medical Sciences to promote implementation of innovation and high-quality development of traditional Chinese medicine (with photos) (11 September 2024)
- The reference DNA sequence library of project “Reference DNA Sequence Library for Chinese Materia Medica (CMMRSL)” has been updated in June 2024 (21 June 2024)
- GCMTI of DH extended scope of accreditation under the Hong Kong Laboratory Accreditation Scheme (HOKLAS) to “Identification of Chinese Materia Medica by DNA Sequencing” (11 June 2024)
- DH signs Co-operation Agreement on Research of Chinese Medicines Standards and on Chinese Medicines Herbarium with National Institutes for Food and Drug Control of National Medical Products Administration (with photos) (9 May 2024)
- DH steps up enforcement to combat illegal sale of drugs and Chinese herbal medicines in retail shops for Labour Day Golden Week of Mainland (30 April 2024)
- Secretary for Health praises DH's new dedicated Digital Herbarium for Chinese Medicines website (with photo) (26 March 2024)
- The 21st to 30th monographs of project “Identification of Tiny Seed and Fruit Types of Chinese Materia Medica” have been published in February 2024 (28 February 2024)
- Recall of Chinese herbal medicine exceeding limits for aflatoxins (with photo) (21 February 2024)
- DH hosts 13th Meeting of International Advisory Board on Hong Kong Chinese Materia Medica Standards (with photos) (16 January 2024)
- DH investigates suspected illegal sale and possession of unregistered proprietary Chinese medicine (with photo) (2 January 2024)
- GCMTI of DH won the Testing and Certification Manpower Development Corporate Award (Platinum Award) (4 December 2023)
- The reference DNA sequence library of project “Reference DNA Sequence Library for Chinese Materia Medica (CMMRSL)” has been updated in November 2023 (30 November 2023)
- DH investigates case of digitoxigenin poisoning and reports recall and investigation progress of one batch of herbal medicine labelled as "Rhizoma Bletillae" (14 November 2023)
- DH endorses recall of one batch of herbal medicine suspected to be confused with "Rhizoma Bletillae" by licensed Chinese herbal medicines wholesaler (with photo) (3 November 2023)
- Director of Health meets delegation of National Administration of Traditional Chinese Medicine and China Academy of Chinese Medical Sciences (with photos) (1 November 2023)
- A brand new TV programme titled “Hong Kong Medicinal Street”, jointly produced by the DH and RTHK, will be broadcasted on 5th November and 12th November 2023 (20 October 2023)
- DH investigates suspected illegal possession of unregistered proprietary Chinese medicine (with photo) (8 September 2023)
- The 11th to 20th monographs of project “Identification of Tiny Seed and Fruit Types of Chinese Materia Medica” have been published in August 2023 (28 August 2023)
- DH endorses recall of Chinese herbal medicines contaminated with solanaceous alkaloids by licensed Chinese herbal medicines wholesaler (with photo) (17 August 2023)
- DH investigates two cases of solanaceous alkaloid poisoning (15 August 2023)
- DH investigates case of undeclared Western drug ingredients detected in cream product prescribed by listed Chinese medicine practitioner (27 July 2023)
- DH investigates suspected illegal possession of unregistered proprietary Chinese medicine (5 June 2023)
- The reference DNA sequence library of project “Reference DNA Sequence Library for Chinese Materia Medica (CMMRSL)” has been updated in June 2023 (1 June 2023)
- Recall of Chinese herbal medicines exceeding limits for aflatoxins (28 February 2023)
- The 6th to 10th monographs of project “Identification of Tiny Seed and Fruit Types of Chinese Materia Medica” have been published in February 2023 (27 February 2023)
- DH hosts Standing Committee Meeting and International Symposium of Western Pacific Regional Forum for the Harmonization of Herbal Medicines (with photos) (23 February 2023)
- Health Bureau and Department of Health meet National Medical Products Administration delegation (with photos) (13 February 2023)
- Consultation for the Amendment of Schedule 1 and Schedule 2 of the Chinese Medicine Ordinance (Cap. 549) (13 January 2023)
- First 5 monographs of project “Identification of Tiny Seed and Fruit Types of Chinese Materia Medica” have been published in December 2022 (1 December 2022)
- Five monographs of project “Study on the Identification of ZiziphiSpinosaeSemen and Its Commonly Confused Species” have been published in November 2022 (30 November 2022)
- The reference DNA sequence library of project “Reference DNA Sequence Library for Chinese Materia Medica (CMMRSL)” has been updated in November 2022 (30 November 2022)
Latest Activities
- The Chinese Medicine Online Webinar will be held on 27 March 2025